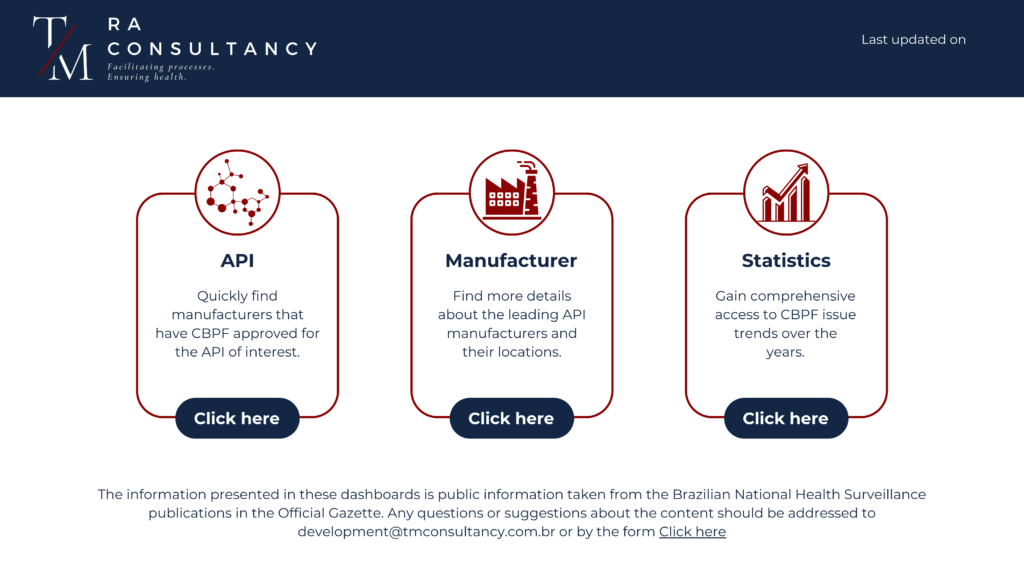
We are pleased to announce that we will soon be launching a new dashboard dedicated to the publications of Good Manufacturing Practices Certificates (CBPF) issued by Anvisa. This new resource has been developed to facilitate access to information and quickly find specific API and certified manufacturers.
What do we offer?
1. Simplified Access: The dashboard will provide an intuitive and easy-to-navigate interface, allowing users to quickly find API manufacturers and information related to Anvisa’s CBPF publications.
2. Customizable Filters: Users will be able to use customizable filters to search for specific manufacturers, API categories, publication dates, and other relevant criteria, ensuring an efficient and targeted search.
3. Real-Time Updates: With our dashboard, you will have access to real-time updated information, ensuring that you are always aware of the latest publications and changes in good manufacturing practices certificates.
Benefits of the New Dashboard
Efficiency and Speed: Quickly find the information you need, saving time and resources in the search for API manufacturers.
Transparency: Access clear and detailed data about manufacturers and their certifications, promoting greater transparency in the sector.
Convenience: We centralize all the information in one place, making navigation and data consultation more convenient for all users.
Stay Tuned!
The dashboard will be available to all users soon. Stay tuned for our updates and be one of the first to experience this new search tool that promises to transform the way you access information about Anvisa’s CBPF and API manufacturers.
Contact us
For more information about the dashboard launch or to clarify any questions, please do not hesitate to contact us.